Nithya - a collagen boosting injectable from horse tendons - the next big thing?
/Nithya, an injectable derived from horse tendons, is being used in the UK in leiu of Botox for wrinkle reduction.
Nithya is a Hindi girls name meaning "always" or "eternally". It's bening touted as a year-long replacement for Botox with a different, and better, treatment mechanism.
There's an article from the UK discussing Nithya as Botox replacement. Link
Described as a 'truly unique form of pain-free anti-ageing treatment', Nithya, which is offered at Vida Aesthetics, is the first skin rejuvenation injectable made from equine-sourced protein available in the UK...
According to the clinic, results are long-lasting, and unlike wrinkle reduction treatments like Botox, which paralyse the muscles and dermal fillers, Nithya apparently works 'in harmony' with your natural tissue structure. The clinic say it lasts a year, whereas Botox lasts around four months.Eddy Emilio, Director of Vida Aesthetics, who is pioneering the treatment, says the launch of Nithya is already proving popular with his clients.'This exceptional collagen-boosting product is proven to be safe, has had no reported side effects, is hypoallergenic and gives excellent results. We truly believe this could rival the results of established but overused anti-ageing cosmetic treatments,' he said.'This is the only commercially available Type I collagen intended for aesthetic use in the country, and we’re already getting rave reviews from cosmetic doctors thanks to its excellent results and numerous areas it can improve the appearance of.'The idea of using protein sourced from horses may seem quirky, but we’re certain this is the future of anti-ageing!'
Described as a 'truly unique form of pain-free anti-ageing treatment', Nithya, which is offered at Vida Aesthetics, is the first skin rejuvenation injectable made from equine-sourced protein available in the UK.
The £250 skin plumping treatment is apparently designed to holistically improve the production of new collagen, helping to ease fine lines around the eyes and improve facial volume in areas such as the cheeks.
Described as a 'truly unique form of pain-free anti-ageing treatment', Nithya, which is offered at Vida Aesthetics, is the first skin rejuvenation injectable made from equine-sourced protein available in the UK.
According to the clinic, results are long-lasting, and unlike wrinkle reduction treatments like Botox, which paralyse the muscles and dermal fillers, Nithya apparently works 'in harmony' with your natural tissue structure. The clinic say it lasts a year, whereas Botox lasts around four months.
Eddy Emilio, Director of Vida Aesthetics, who is pioneering the treatment, says the launch of Nithya is already proving popular with his clients.
'This exceptional collagen-boosting product is proven to be safe, has had no reported side effects, is hypoallergenic and gives excellent results. We truly believe this could rival the results of established but overused anti-ageing cosmetic treatments,' he said. 'This is the only commercially available Type I collagen intended for aesthetic use in the country, and we’re already getting rave reviews from cosmetic doctors thanks to its excellent results and numerous areas it can improve the appearance of.
'The idea of using protein sourced from horses may seem quirky, but we’re certain this is the future of anti-ageing!'
The Vida Aesthitic clinic has some before and after photos posted here
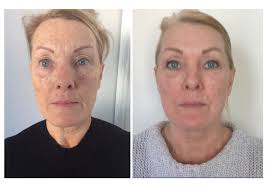
From Vida Aesthetics site:
(Nithya) Is a Class III medical device. It is a heterologous Type I collagen powder which stimulates the production of new fibroblasts to create native Type III collagen, a process that is fundamental in cosmetic medicine and anti-aging treatments. In the form of a lyophilized collagen patch, this product has been used for almost 30 years and is still successfully used today to affect the healing of skin ulcers, open wounds, scars and bedsores.
Each package consists of a glass bottle containing equine type I collagen in powder form, sterile and pyrogen-free.
Is safe, with no reported side effects, is hypoallergenic and gives excellent results. Used in combination with lidocaine, the surgery procedure is virtually painless and is the only commercially available Type I collagen intended for aesthetic surgery. The product bears the CE 0373 mark.
It produces the optimal conditions to restore connective tissue. It supplements dermal bio-revitalization and assists the regeneration of connective tissue in the dermis proving perfect conditions for the physiological neo-formation of collagen. Can be used for body and facial chrono- and photo-aging treatments.
Packaged for single use and must be placed in suspension immediately before use in WFI (Water For Injection) at a ratio of a vial of collagen to 5 ml of WFI. Once in suspension, the product should be used immediately and any remaining following the treatment should be discarded. Using the appropriate size needles from 32G x 2 mm up to 30G x 4 mm, the product is administered through intradermal infiltration by medical practitioners licensed to carry out such treatments. The technical skill of the practitioner is key to the success of the treatment so it is recommended that the practitioner receives specific training on the mesotherapic injection technique.
Vida Aesthetics is also offering Nithya injectable training: Link





